
Albert Einstein (1879-1955): “The whole of science is nothing more than a refinement of everyday thinking.”
“Science Book” belongs to Arts, Reviews and Current Affairs, a subsidiary page of the main site.
Concepts will span all the key scientific disciplines including the traditional sciences of physics, biology and chemistry while other areas including biotechnology, the earth sciences and information technology will also be examined. A series of Science Books covering these subjects can be found on this site.
INTRODUCTION
Science is a powerful tool for understanding nature. Amazingly so. For instance, it has revealed compelling evidence that the universe was born in a fiery explosion 13.7 billion years ago. Scientists have cracked the genetic codes of complex life, eradicated diseases like smallpox and scientific thinking provides the best tools in the box when it comes to problem solving.
For simplicity, this web page will be built slowly and methodically. There will, inevitably, have to be some narrowing down of the topics, but the page(s) will incorporate all the must-have basics for comprehensive understanding.
Modern adventures in science remind us that the subject is not just about rote learning long-established theories – a scientist’s real job is to find out things we don’t already know. Most of the matter in the universe remains stubbornly unidentified. The real buzz of science lies in detective work, which will keep future scientists busy for a long time to come.
Motion – In physics, the motion of an object is described in terms of quantities such as velocity, acceleration and displacement, the distance of a moving object from its origin. Velocity is a vector quantity, specifying not only an object’s speed but also its direction, while force is a measure of the push or pull that an object needs to change its velocity, resulting in acceleration – the rate of change of velocity over time.
Newton’s laws of motion define the relationship between force and acceleration for everyday objects such as cars and aircraft. Momentum is defined as the product of an object’s mass and its velocity. It is a “conserved quantity”, so that in the absence of any other influences, two snooker balls that bounce off each other will have the same total momentum before and after colliding.
An object’s kinetic energy is equal to half its mass multiplied by the square of its speed. This quantity measures the work needed to accelerate the object to its given speed from rest.
Newton’s laws of motion – Isaac Newton’s three laws of motion, first published in 1687, describe the relationship between a force acting on a body and the body’s motion due to that force.
The first law says that a body moving with a given speed will maintain that speed in a straight line unless a force acts upon it – no force means no acceleration. The second law states that a force [F] will make a body accelerate by an amount [a] that’s inversely proportional to its mass [m]: F=ma. The third law states that whenever one body applies a force [the “action” force] to a second one, the second one simultaneously applies an equal and opposite “reaction” force on the first.
Newton showed that these laws neatly explain the orbits of the planets around the Sun when combined with Newtonian gravity. But they are not valid for objects moving at very high speeds or in very intense gravitational fields, when relative theory is required.
Centripetal and centrifugal forces. A centripetal force is one that makes a body move in a curved path. Gravity is an example of a centripetal force in Newtonian gravity, making a planet orbit a star by continually accelerating the planet towards the star at the orbit’s centre. Without this centripetal force, the planet would fly off into space in a straight line.
When you whirl a tennis ball over your head on a string, the ball feels a centripetal “pull” force. The centripetal force is often confused with the centrifugal (outward) force, which can be a “fictitious” force. It accounts for the sense of being pushed outwards when looping-the-loop on a roller coaster.
The centrifugal force can also be a reaction force to a centripetal force, according to Newton’s third law of motion. In the case of a tennis ball on a string, the whirling ball exerts an outward centrifugal force on the person spinning it. The outward centrifugal force is caused by the ball’s tendency to keep going in a straight line. It’s synonymous with inertia.
Newtonian gravity. Isaac Newton’s law of universal gravitation, published in 1687, was the first clear mathematical description of how bodies such as planets and stars attract each other under their mutual gravitational pull.
Newton’s inspiration for the theory came from watching an apple falling from a tree. A falling apple accelerates towards the ground, so Newton reasoned from his laws of motion (see above) that there must be a force, which he called gravity, acting on the apple. This force might have a huge range and could also be responsible for the orbit of the Moon around the Earth, if the Moon had just the right speed to remain in orbit despite constantly “falling” towards the Earth.
Newton went on to show that the gravitational force between two massive objects is directly proportional to the product of their masses and weakens with the square of the distance between them. But troublingly, the theory didn’t explain why the force was transmitted across empty space. This problem is resolved in Einstein’s general relativity theory.
. See Quantum Leaps: Sir Isaac Newton
Special relativity. Special relativity is the theory of motion published by Albert Einstein in 1905. Einstein developed it from two basic principles: the laws of physics must be the same for any observer moving at a constant velocity, and the speed of light is always the same, regardless of the speed of the light source.
Relativity abandons the idea that it’s possible to have a universal standard of time and space. Instead, the length of an object or time interval depends on who is measuring it. Take the case of a train moving at close to the speed of light relative to an observer. The observer would perceive the train to be shorter than the passengers on board would measure, while the observer would see a clock on the train run slow.
This is not just an illusion – measurements show that unstable particles moving fast through Earth’s atmosphere decay much more slowly than they do at rest in a laboratory. Special relativity forbids massive objects from travelling as fast as the speed of light in a vacuum, which would require an infinite amount of energy.
In the video below, Sabine Hossenfelder explains time-dilation in respect to acceleration.
General relativity. General relativity is Einstein’s theory of gravity, which had been developed by 1915. Unlike Newtonian gravity, Einstein’s theory views gravity as a natural upshot of the geometry of curved space, and ditches the notion that gravity is “action at a distance”. Large masses like planets move in response to the curvature of space-time, distorted by mass itself. Matter tells space how to curve; curved space tells matter how to move.
It’s difficult to visualise in three dimensions, but it helps to imagine a star’s mass making a depression in a two-dimensional sheet. A nearby planet would be forced to curve around it like a ball in a roulette wheel.
Some predictions of general relativity are different from those of Newtonian gravity. Although both theories predict that the Sun’s gravity bends light from background stars, which can be measured when sunlight is blocked during a solar eclipse, Einstein’s theory predicts twice as much deflection as Newton’s. Measurements show general relativity is correct on this, and it has passed all other tests so far with flying colours.
. See Quantum Leaps: Albert Einstein
Temperature and pressure. Temperature is a measure of how hot an object is, which reflects the amount of kinetic energy in its molecules. For common purposes, most countries measuring temperatures use the Celsius scale of temperature, which sets water’s freezing point at zero degrees (0°C) and its boiling point at 100°C. The US uses the Fahrenheit scale, in which water freezes at 32°F and boils at 212°F.
Matter can be cooled by reducing the kinetic energy of its molecules, but the laws of thermodynamics predict that there is a minimum possible temperature, which turns out to be -273.15°C (-459.67°F). This is “absolute zero”, where particles would theoretically be motionless.
Pressure is the force exerted by one substance on another substance, per unit area. The pressure of a gas is the force the gas exerts on the walls of its container. The standard unit of pressure is the pascal (1 newton of force per m²). The typical air pressure at sea level on Earth is about 100,000 pascals.
The speed of molecules increases with temperature.
Heat transfer. Heat can be transmitted through matter in three ways: conduction, convection and electromagnetic radiation. Unlike conduction and convection, radiation can transmit energy across empty space.
Conduction is the mechanical transfer of heat through matter from a warm part to a cooler one without any bulk motion. In gases and liquids, heat conduction occurs due to collisions and diffusion of molecules during their random motion. In solids, molecules conduct heat by vibrating against each other or when free electrons carry kinetic energy from one atom to another. Metals are the best conductors of heat.
Liquids and gases also transfer heat in convection currents, which involve bulk fluid motion. For instance, a hot bubble of gas in the Sun’s atmosphere can carry heat into a higher, cooler layer before cooling and sinking. Heat transfer can also occur when radiation carries energy between one object and another. For example, sunlight heats the Earth by making molecules in the Earth’s atmosphere and surface vibrate.
Brownian motion. Brownian motion describes the jittery, random motion of relatively large particles suspended in a fluid or gas, such as smoke particles in air. It is named after Robert Brown, a Scottish doctor and botanist who studied it in detail in 1827.
Dr Brown noticed that pollen grains in water jiggled about, following zigzag paths. Later in 1905, Albert Einstein showed that this Brownian motion can be mathematically predicted by assuming these large, suspended particles are constantly bumped by smaller fluid molecules moving due to their own thermal energy. One prediction was that the displacement of a suspended particle from its origin over time should be proportional to the square root of the time elapsed.
Experiments by French physicist Jean Perrin soon confirmed that Einstein’s predictions were correct, indirectly proving that molecules and atoms exist, despite the fact they were too small to be seen directly. This might seem obvious now, but it was still common at the time to believe that matter was not grainy and could be divided indefinitely.

Work and energy. Work refers to an activity involving a force and a movement, while energy is the capacity for doing work – a bit like a “currency” that gets used up in the process. In the context of a moving object, the work done by a force equals the force multiplied by the distance moved.
In the context of thermodynamics, work has a more complex definition. It refers to energy transferred to a gas, for instance, but only if that energy causes a macroscopic change to the gas, perhaps making it expand its volume against an external pressure. It doesn’t include the input of heat energy if the heat merely increases the microscopic thermal motions of particles.
The work done to compress a gas in a container with a movable piston is approximately equal to the gas pressure times the volume change. The change in internal energy of a gas is equal to the heat added minus the work done by the gas. This is one way of stating the first law of thermodynamics.
Laws of thermodynamics. The four laws of thermodynamics define the relationships between quantities like temperature and work in “thermodynamic systems” – a loose term for any matter with thermal energy, such as gas molecules in a container.
“Thermal equilibrium” describes the state of two systems in contact with each other, which have no net exchange of energy because they’ve reached the same temperature. The “zeroth law” of thermodynamics says that two systems in thermal equilibrium with a third one must also be in thermal equilibrium with each other. Scientists felt the need to state the intuitively obvious zeroth law after they adopted the other three.
The first law says energy in an isolated system is conserved. Chemical energy might change into kinetic energy, but the total stays the same. The second law states that because energy varies in its quality or ability to do useful work, the entropy of an isolated system – a measure of the energy input that doesn’t do mechanical work – always increases. The third law says minimum entropy occurs at absolute zero.
A presentation by Eugene Khutoryansky who explains entropy and why the second law of thermodynamics is a fundamental law of physics:
Phases of matter. Classically, matter can exist in three phases: solid, liquid and gas. Traditionally, solids are defined as matter with a fixed volume and shape, containing closely packed particles. Liquids keep the same volume but flow to fill the bottom of a container, while gases expand to occupy all available volume.
Phase transitions can occur due to alterations in pressure or temperature. At normal atmospheric pressure, pure water melts from solid ice into a liquid above 0°C [32°F] and boils into water vapour at 100°C [212°F]. The energies of individual water molecules in a boiling kettle are not identical but follow a bell curve, which means liquid and gas phases can co-exist. At the so-called triple point of a substance, all three phases can co-exist. For instance, water ice, liquid and vapour can mingle in a container at 0.01°C [32.02°F] at very low pressures.
Plasma, a searingly hot ionised [electrically charged] gas, is often called a fourth state of matter. It streams out from stars like the Sun into interstellar space. More exotic states of matter include Bose-Einstein condensates. These are a peculiar state of matter that some particles form when they all crash down into their lowest possible energy states at temperatures close to absolute zero. The condensates are an interesting window on the physics of quantum mechanics, because they illustrate quantum effects on a macroscopic scale.
Surface tension. Surface tension results from the inward pull of molecules on the surface of a liquid, making them adopt the smallest surface area possible. It effectively makes the surface stronger, allowing a small object such as a sewing needle to effectively “float” on water, even though the object may be much denser than the liquid.
In the bulk of a liquid, molecules face a tug of war in which they’re pulled equally in all directions by neighbouring molecules, so the forces on them cancel out. But surface molecules lack upward force, so they’re pulled together and down, making the surface contract to its minimum size.
Surface tension holds water droplets together and would make them spherical in the absence of other forces such as gravity, because a sphere has the smallest surface to volume ratio. Many animals take advantage of surface tension on ponds. Common insects called water striders, or pond skaters, rely on it to walk on water and sense vibrations from nearby prey using sensitive hairs on their legs and bodies.
Archimedes’ principle. Archimedes’ principle states that the buoyant force on an object submerged in a fluid (liquid or gas) is equal to the weight of the fluid that the object has displaced. It implies that an object will sink in a fluid if its average density is greater than that of the fluid.
Archimedes was a Greek scientist and engineer who lived during the 3rd century BCE. Later historians suggest that he was tasked with determining whether a crown supposedly crafted from pure gold also contained some cheaper silver. While taking a bath, Archimedes noticed that the water level rose when he got in, and realised that by placing the crown in water and measuring the displaced water volume, he could establish the volume of the crown, and thus calculate its density and purity without damaging it.
Legend has it that Archimedes then ran down the street naked shouting “Eureka!”, Greek for “I have found it”. His principle explains why ships float and why hot-air balloons rise – warm air within a balloon is less dense than the cooler air outside.

. See Quantum Leaps: Archimedes…
Fluid dynamics. Fluid dynamics is the science of how fluids (both liquids and gases) flow. It’s essential for many practical applications, including the design of efficient aircraft, ships and oil pipelines as well as weather forecasting.
A boat moving through water encounters two main kinds of resistance – inertial forces from the water (effectively the water’s resistance to motion) and viscosity or stickiness. In fluid dynamics, the “Reynolds number” expresses the relative importance of these factors in flows across a surface, such as a ship’s hull or a pipeline. A low Reynolds number gives smooth fluid motion, while turbulent flow with chaotic eddies and vortices occurs at high Reynolds numbers.
One key concept in fluid dynamics is the Bernoulli effect: the faster a fluid flows, the lower its pressure. The curved upper surfaces of aircraft wings are shaped to force air to follow a longer path over the top of the wing, speeding it up. This lowers pressure above the wing and creates a net upward force, or lift.
Wave types. A wave is a disturbance that propagates through empty space or a medium such as air or water, usually transporting energy as it travels.
In “traverse waves”, the disturbance is at right angles to the wave’s direction of motion. Electromagnetic radiation, including visible light, is a form of traverse wave in which magnetic and electrical fields oscillate at right angles to the wave’s direction of travel. In “longitudinal waves”, the disturbance is parallel to the waves direction. These include sound waves in gases and liquids. Water waves are an example of a wave that is both traverse and longitudinal – a floating cork will move in a circle as a wave washes past it.
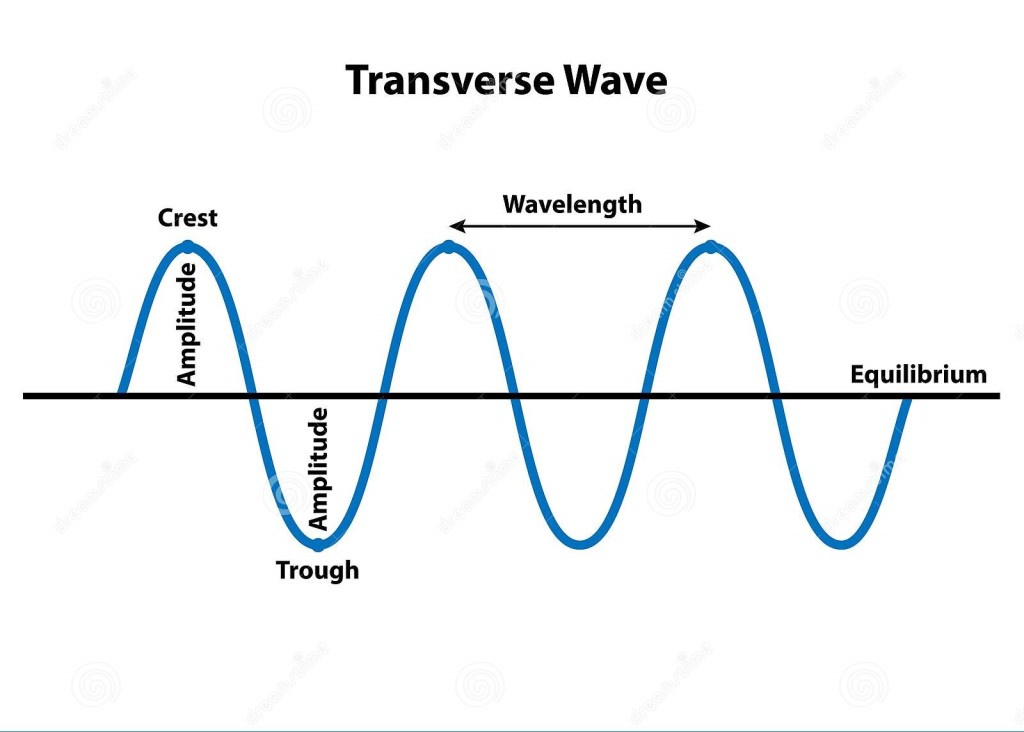
Waves are characterised by their wavelength – the distance between peaks or compressions), frequency (the rate at which waves pass a given point) and amplitude or intensity. Standing or stationary waves occur when waves are held in a fixed position – for instance, when a guitar string vibrates. Such waves always involve a whole or half-number of waves, and hence the length of the string determines the wavelengths it can maintain.
Sound waves. Sound waves are pressure oscillations that propagate through a gas, liquid or solid – sound can’t travel in empty space. In gases and liquids, sound is a longitudinal wave but traverse sound waves can pass through solids.
People hear sound because it makes our ear drums vibrate. These vibrations pass through the inner ear to nerve cells that send signals to the brain, which interprets them as sound. A higher wave frequency means that the air pressure fluctuation switches back and forth more quickly, and we hear this as a higher pitch. Human hearing is normally limited to frequencies between 20 and 20,000 hertz (repeating wave cycles per second) and the upper limit tends to drop with age.
The speed of sound depends only on the transmitting medium. In air with a temperature of 20°C [68°F] at sea level, sound travels at about 343 m/s [767 mph]. The intensity of sound is measured in decibels, with a typical conversation registering about 60 decibels and motorbike engines topping 100 decibels.
Doppler effect. The Doppler effect describes changes in the frequency of waves depending on how the wave source is moving relative to an observer. It explains why the siren of a fire engine sounds higher in pitch as it comes towards us, then sounds lower after the vehicle has driven past.
When a source of sound waves moves towards an observer, each successive wave emitted comes from a position closer to the observer, who hears it more quickly due to the shorter travel time. Effectively, the waves bunch together creating an increase in frequency. Conversely, when the wave source moves away, successive waves are emitted from greater distance. The waves stretch out, making their frequency drop.
The effect is named after Austrian physicist Christian Doppler, who described the effect for light waves in 1842. Frequency also determines the colour of light, so the Doppler effect alters the colour of a light source approaching an observer or receding at very high speed – a green light appears more blue when approaching and more red when receding.
Electric charge. Electric charge is a property of many standard model particles, including the electron. This property makes them feel a force from other charged particles. Electrical charge can be either negative or positive, with negatively charged particles attracting positively charged ones while repelling their own kind.
The unit of electric charge is called the coulomb [C]; 1 coulomb is the charge transported per second by an electric current of 1 ampere. The negative charge of an electron is -1.602 x 10^-19C. For simplicity, the electron charge is often donated as -1, while that on a positively charged proton is +1.
Electric charge plays a pivotal role in our very existence, allowing solid structures like the Earth, buildings and animals to exist. Atoms are mostly empty space, but they don’t fall through each other due to repulsion between electrons in neighbouring atoms. Charged particles zinging around in the Sun’s atmosphere also play a crucial role by generating the radiation that keeps our planet’s surface warm and hospitable.

Electric current. Electric current is the flow of electric charge, carried by moving electrons. A current flows through a conducting material such as copper wire when it is connected to the positive and negative terminals of a battery (which applies an electrical potential difference or voltage). Electrons in the wire then drift towards the positive terminal.
National electricity grids deliver alternating electric current that reverses periodically, usually 50 or 60 times a second. The unit of electric current is the ampere; 1 amp is the flow of 1 coulomb of charge per second.
Electrical resistance is the extent to which a material resists the flow of electric current, and is measured in ohms. Metals such as silver and copper have low resistance, allowing current to flow easily, while plastics and wood have high resistance, making them poor conductors. The current in a wire is equal to the voltage applied across it divided by the resistance, while electrical power – the energy transmitted per unit time – is the product of the voltage and current.

Magnetism. Magnetism is a property of materials that makes them experience a force in a magnetic field. It explains why iron filings line up in ordered patterns near a bar magnet for instance, and why fridge magnets stick to fridges.
Bar magnets are strips of magnetised metal, usually iron, that form a “dipole” field with a north and south pole. Opposite poles attract each other, while like poles repel. The magnetic field of a permanent magnet arises because electrons inside it generate their own tiny magnetic fields due to an intrinsic property called spin, and in materials like iron, the spins of unpaired electrons tend to line up.
Scientists have known since the early 1800s that there is a deep connection between magnetism and electric current. For instance, an electric current flowing through a coil of wire creates a dipole magnetic field similar to that of a bar magnet. Modern electromagnets have achieved record-breaking magnetic fields of 35 teslas, where 1 tesla is about 20,000 times stronger than the Earth’s magnetic field. Geomagnetism, which refers to the Earth’s magnetic field, is part of Earth Science and will be covered in Science Book later.


Induction and capacitance. Induction occurs when a conducting material moves through a magnetic field. In 1831, the scientist Michael Faraday showed that this makes an electric current flow through the conductor. Induction underpins the operation of electrical equipment from electric motors to electricity generators.
Dynamo generators convert rotational motion produced by a turbine, for instance, into electricity, while electric motors do the opposite, creating rotational motion from an electric current. In both cases, the motion, magnetic field and electric current are all perpendicular to each other in directions illustrated by left-hand and right-hand rules, memory aids invented by British engineer John Ambrose Fleming.
Electrical circuits also have “self-inductance” – changes in current in a wire will generate a changing magnetic field, which in turn induces a current. Inductors are electrical components designed to store energy in induced magnetic fields, while “capacitors” store energy in electrical fields. In simple capacitors, opposite electric charge builds up on two parallel plates.


Electromagnetic radiation. Electromagnetic radiation is a form of energy that can travel through empty space and includes visible light. It includes gamma rays, which can cause radiation sickness by damaging cells, and radio waves, which are vital for wireless communication technologies.
Electromagnetic radiation is a transverse wave [see above] consisting of oscillating electric and magnetic fields. In a vacuum, the waves always travel at 300,000 km/s [671 million mph], but their wavelengths vary enormously. Gamma rays have tiny wavelengths, often smaller than an atom, while radio waves can be thousands of kilometres across.
We can only see a small part of the electromagnetic spectrum – visible light spanning the rainbow of colours from violet to red. Visible light passes through the Earth’s atmosphere and reflects off objects allowing us to see them. Many insects, fish and birds can also see ultraviolet radiation, which attracts bees to flowers. Gamma rays are much more penetrating and can pass through several centimetres of lead.

Photons. The photon is the quantum of electromagnetic radiation and the basic “unit” of light. In a vacuum, photons all move at the same speed: 300,000 km/s.
Light seems to have a split personality – it can be thought of both as a wave and a stream of particles. Albert Einstein nailed the case for the particle-like nature of light when he explained the “photoelectric effect”, in which light shining onto a piece of metal makes the metal eject electrons. Strangely, a dim blue light has this effect, but a red light, no matter how bright, does not. Einstein realised that this is because light consists of discrete energy packets. An individual photon of blue light has enough energy to dislodge a single electron from a metal, but red photons do not, regardless of how many there are.
Photons have no mass or electric charge, but they carry momentum. The energy of a photon of light is proportional to the light’s frequency, with gamma-ray photons carrying billions of times more energy than radio photons.
Lasers. Laser light differs from ordinary light because it is much more organised – a bit like a marching army compared to a bustling crowd. While light from a light bulb contains many wavelengths, laser light contains only one. It forms a much narrower beam and is “coherent”, meaning the waves all travel in lockstep, with their crests and troughs lined up.
Lasers [short for Light Amplification by Simulated Emission of Radiation] were first developed in the 1960s. Laser light is produced when atoms or molecules are excited to higher energy levels in a cavity, then zapped with photons of a specific energy that the hyped-up particles can emit to relax back to their normal state. This makes the particles emit clones of these photons, with exactly the same properties, in a chain reaction to create a laser beam.
Lasers have a host of everyday applications, such as reading information in DVD players and barcode scanners, and as an instrument for hospital surgery. Future gamma-ray lasers could focus a million times more energy than the current generation.
. Light from a normal source is a mix of wavelengths and frequencies diverging from source
. Light rays from a onochromatic light source have identical wavelengths but are not in lockstep, and diverge from source
. Light rays from a coherent laser light source are monochromatic and tightly aligned, with waves in lockstep
Reflection and refraction. In many situations, light can be thought of simply as a transverse wave [see above] that travels in a straight line until it encounters an obstacle. Light reflects in a simple way off smooth surfaces like mirrors, obeying a law that the angle of reflection [measured from the “normal”, a line perpendicular to the mirror] equals the angle of incidence or approach.
Refraction is the process that happens when light travels from one transparent medium, such as empty space or air, into another one, such as water. Light travels slower in water than in a vacuum, and this change of speed is responsible for the bending of light, or refraction. The ratio of the vacuum speed to the water speed is called the “refractive index” of water, which is about 1.33.
Light bends towards the normal when passing into a denser medium and away from the normal when entering a less dense one. Glass lenses in spectacles and optical instruments like telescopes are specially shaped to refract light rays onto the required paths for correcting poor vision or focusing starlight.


Diffraction. Diffraction describes the way waves bend around obstacles they encounter. A classic example is the way that water waves fan out when long, straight wavefronts encounter a narrow opening, then emerge as small circular waves beyond it.
The effect is easy to demonstrate by creating ripples in a tray of water containing a barrier with two gaps in it. Straight waves approaching the barrier create small circular waves beyond the two gaps, and as they move outwards they undergo interference, with crests amplifying each other while crests and troughs cancel out. Light diffraction patterns can be unintuitive and are best seen with laser light. The diffraction pattern of a square hole is cross-shaped, while a circular aperture produces a series of concentric circles.
Diffraction by water droplets or ice crystals in thin clouds sometimes creates a beautiful bright ring around the Sun or the Moon. But diffraction is largely a nuisance to optical instrument designers, setting fundamental limits on the clarity of images taken by cameras, microscopes and telescopes.
Polarisation. Polarisation is a property of transverse waves that are restricted in their directions of oscillation. It’s most frequently discussed in the context of electromagnetic radiation, including normal light, which can be polarised using a filter that only transmits light beams “waving” in one plane.
Light consists of electric and magnetic fields oscillating perpendicular to each other, but the electric fields in ordinary light from the Sun or a torch oscillate in all possible planes. A linearly polarised light beam is one in which the electric field oscillations are restricted to one plane. It’s also possible to create circularly polarised light, in which the electric field oscillations continually rotate like the thread of a corkscrew as the light beam moves through space.
Light reflected from surfaces such as a flat road or smooth water tends to be horizontally polarised. Polarised sunglasses reduce reflected glare using filters that contain long-chain molecules. These preferentially absorb horizontally polarised light, so that only the vertical component passes through.
In the diagram:
. Normal unpolarised light oscillates in multiple planes and in all directions
. The polarised filter acts as a narrow grille
. Emerging polarised light oscillates in just one plane

Interference. Interference occurs when waves overlap. If you drop two stones in a puddle and watch the ripples spread out, you’ll see them combine to form a distinctive pattern in which coinciding wave crests have amplified each other in “constructive interference”, as having matching troughs, while peaks and troughs cancel each other out in “destructive interference”.
A thin film of oil on water can create colourful light interference patterns when sunlight reflects from the top of the oil as well as the oil-water boundary. The two reflections have followed different path lengths, so when they recombine, they interfere in a constructive or destructive way depending on the light’s particular wavelength, or colour. This makes white light fan out into a rainbow of colours that changes with viewing angle. Light reflecting off the many grooves of a shiny CD or DVD creates colourful interference in a similar way.
Sound interference is noticeable when two tones have an almost identical pitch. This creates a warbling effect called beating due to constructive and destructive interference.
In the diagram (known as the dual-slit experiment):
. Monochromatic light source produces light of a single wavelength
. Barrier with dual slits
. Constructive interference where peaks meet peaks and troughs meet troughs
. Destructive interference where peaks meet troughs
. Interference pattern formed on screen

Quantum mechanics. Quantum mechanics is a branch of physics that describes the strange behaviour of matter and energy on the tiniest scales. Scientists developed it in the early 20th century when experiments revealed cracks in classical physics. It was clear that electrons orbited an atom’s nucleus, for instance, but if they did so like planets orbiting the Sun, they should fall into the nucleus in a split second – which obviously doesn’t happen.
Quantum mechanics invokes ideas like the Heisenberg uncertainty principle to explain the anomalies of tiny realms. One key concept is that properties of particles, such as the energies of electrons in an atom, can only change by discrete amounts – they are said to be “quantised”.
The quantum world is strangely unpredictable. Everyday experience suggests you could take an electron, apply a force to it, then predict where the electron will be a second later. Quantum mechanics says this is impossible. You can estimate the likelihood of the electron reaching a given place, but until you measure its position, it’s in all possible places at once.
In the “Bohr model” of an atom, electrons orbit the atomic nucleus in quantised “orbitals” – they can only move between orbitals by absorbing or emitting energy.

Wave-particle duality. Wave-party duality describes the way matter and energy on the smallest scales can be viewed as both particles and waves. In normal life, we expect moving particles to behave like small projectiles, while waves spread out like ripples on a pond. In quantum mechanics, this distinction is blurred.
Electrons display this duality in the “dual-slit experiment” [see above]. When electrons emerging from a source shine through two slits onto a phosphorescent screen, bright and dark bands form due to interference analogous to that seen in light waves. What’s more, even if the source is calibrated to produce just one electron at a time (which according to classical physics should only pass through one slit or the other, and hence only hit one of two areas of the screen) the interference pattern still builds up over long periods of time.
Bizarrely, the interference vanishes if the experiment is modified to detect which slit each electron is passing through – it’s impossible to observe particle-like positional information and wave-like interference patterns simultaneously.

Uncertainty principle. The Heisenberg uncertainty principle underlines the fuzziness of the quantum world. It states that certain pairs of properties, such as the position and momentum of a particle, can’t both be determined with exact precision at the same time. The more precisely you know the particle’s position, the less precisely you can know its momentum.
In 1927, the German physicist Werner Heisenberg published the principle, which stems from a particle’s wave-like nature.
The only wave with a definite position is concentrated at a single point, but such a wave has an indefinite wavelength, meaning indefinite momentum. Conversely, the only wave with a precise wavelength is infinitely long and has no definite position. So there are no states that simultaneously describe a particle’s exact position and momentum.
Heisenberg’s uncertainty principle quantifies this vagueness – the product of the uncertainty in position and momentum must be greater than or equal to “Planck’s constant” h [a tiny number equal to 6.6 x 10^-34 joule seconds] divided by 4π.
Schrödinger’s cat. Schrödinger’s cat is a thought experiment proposed by an Austrian physicist, Erwin Schrödinger, in 1935. He wanted to highlight a problematic paradox in quantum mechanics, that the properties of particles can’t be determined until they’re observed. The position of an electron, for instance, is a superposition of all possible positions until it is measured.
The thought experiment questions what would happen to a cat shut in a box with a device containing a radioactive nucleus and a lethal poison. If the nucleus decayed by emitting a particle, that would trigger the poison’s release, killing the unfortunate cat. But quantum theory forbids predictions of when the nucleus will decay. So is the cat both dead and alive until we “measure” its state by opening the box to look at it?
To this day, scientists debate various solutions to this unpleasant paradox. Perhaps the simplest view is that quantum theory doesn’t really create this paradox at all, because it clearly states that the only possible measurements are sensible ones, in this case a dead or alive cat, nothing in between.
Quantum entanglement. Quantum entanglement is a weird effect in which two particles can be set up to “know” what the other one is doing, even when they are separated by thousands of miles and have no way of communicating with each other.
The effect arises in quantum mechanics because it’s possible to link the properties of two particles so that they will always be related. For instance, two light photons can be primed so that their polarisation states are unknown, but when measured will be opposite. The two photons can zoom off into space in opposite directions with undefined polarisations, but when someone later measures the polarisation of one photon, the second one will take on the opposite polarisation. It’s like instantaneous communication, faster than the speed of light.
Albert Einstein was suspicious of theories that allowed quantum entanglement, calling it “spooky action at a distance”. But experiments prove that it really happens. Scientists have successfully transmitted entangled photons between sites on Spain’s Canary Islands 140 km (87 miles) apart.

Casimir effect. In quantum mechanics, the Casimir effect describes the tiny attractive force acting between two parallel, uncharged conducting plates in a vacuum. It arises because a vacuum is not just empty space – it is seething with energy and particles that are constantly popping in and out of existence.
In 1948, the effect was predicted by Dutch physicist Hendrick Casimir, who realised that close metal plates would block out light waves that are too big to fit between them. If the gap was only a few nanometres [billionths of a metre] wide, the energy density outside the plates would be higher than between the plates, creating a pressure to push them together. In a nautical analogy, two large ships that are side by side in windless conditions drift together. The ships cancel waves between them, while waves outside buffet the ships together.
The Casimir-effect can also be a repulsive force, depending on the experimental arrangement. It could one day be useful in nanoscale machinery creating repulsion between components that lets them move without friction. Nanotechnology is molecular engineering which will be explained more fully when Science Book expands into the realm of chemistry.
Superfluids. Superfluids are fluids that have no viscosity, or stickiness, and so move without experiencing friction. In 1962, experiments produced the first superfluid using helium-4, chilled to just 2.17C above absolute zero. Helium-3 also forms a superfluid, but at an even lower temperature.
Superfluids are famous for the odd and strange behaviour. Placed in a beaker, superfluid helium creeps up the sides and over the top. Another strange property is that its spin is quantised – it only rotates at certain allowed speeds. When the container of a superfluid rotates below the liquid’s sound speed, the fluid doesn’t move. Once the container’s speed reaches the sound speed, the superfluid suddenly spins at this speed.
Perfect superfluids also have infinite thermal conductivity. A hot spot in superfluid helium will ripple through it like a sound wave with a speed of around 20 m/s [45 mph]. The name superfluid was coined to echo the term superconductor which describes materials that conduct electric currents without any resistance.
Bose-Einstein condensates. A Bose-Einstein condensate is a peculiar state of matter that some particles form when they all crash down into their lowest possible energy states at temperatures close to absolute zero. The condensates are an interesting window on the physics of quantum mechanics, because they illustrate quantum effects on a macroscopic scale.
In the mid-1920s, Indian physicist Satyendra Nath Bose and Albert Einstein predicted the existence of these condensates. They form particles called bosons, which have integer [whole-number] values of a quantum property called spin.
In 1995, scientists in Colorado produced the first Bose-Einstein condensate by chilling rubidium atoms to nearly absolute zero. The atoms overlap to form a blob that behaves like a single “superatom”. Bose- Einstein condensates might one day have a practical use – lasers have flourished in technologies because they create identical light photons that are easy to control; likewise, Bose-Einstein condensates could fuel technologies that need precise control of identical atoms.
Superconductivity. Superconductivity is a material that can conduct electricity without any resistance. Once set in motion, a current will flow forever in a closed loop of superconducting material.
Superconductivity was first discovered in the element mercury. At a temperature of just 4C above absolute zero, its electrical resistance switches off. Theory suggests low-temperature superconductivity arises because electrons passing through a crystal lattice deform the lattice, creating “troughs” of positive charge that help propel subsequent electrons through the same region.
Known superconductors include metals, polymers and even ceramics. Superconducting coils cooled to very low temperatures are used as superconducting magnets, which can produce extremely strong magnetic fields. They are used in medical scanners and levitating “maglev” trains, which have achieved speeds of more than 580 km/h [360 mph]. The holy grail is to find materials that are superconducting at easily achievable temperatures above 0°C [32°F].
Standard model particles. The standard model describes the most fundamental particles in nature. The tiniest constituents of matter form two families. The first are the quarks, divided into six “flavours” called up, down, charm, strange, top and bottom. These feel the strong force and carry an electric charge of +2/3 or -1/3 times that of the electron. Quarks combine in pairs or threes to form other particles, such as protons and neutrons.
The second group of matter particles are leptons, which don’t feel the strong force. The most familiar lepton is the electron, which has two heavier siblings called the muon and tau. All three have the same electric charge, -1. The final three leptons are “neutrinos”, electrically neutral particles with tiny masses. These stream out from nuclear reactions in the Sun and easily fly straight through the Earth.
The standard model also includes several force-transmitting particles called gauge bosons, including the photon (see above). Scientists suspect there is a “Higgs boson” that endows fundamental particles with mass. Until recently, the Higgs boson remained elusive.

Strong and weak forces. In particle physics, the strong force (also called the strong interaction or strong nuclear force) is a fundamental force of nature. It binds quarks together to form protons and neutrons, and also binds protons and neutrons together inside atomic nuclei. The strong force has a range similar to the size of a typical atomic nucleus.
The weak force, or weak interaction, is another fundamental force of nature. Its range is tiny, only about a thousandth of the size of a proton. Its most familiar effect is beta decay, in which it allows a nucleus to emit an electron or positron and change its overall electric charge. The weak force also initiates hydrogen fusion in stars, and allows one quark to change into another “flavour” of quark.
Scientists are hopeful of eventually crafting a single “theory of everything” that elegantly describes the behaviour of all four forces – strong, weak, electromagnetic and gravitational – within the same mathematical framework.
Antimatter. In a nutshell, antimatter is matter’s nemesis. Every matter particle from the standard model has an antimatter counterpart with equal mass but opposite charge, and when the two meet, they destroy one another on contact.
The British physicist Paul Dirac predicted in the 1920s that there must be a particle in nature that is identical to the electron but with opposite charge. This anti-electron or “positron” was discovered experimentally in 1932. When an electron meets a positron, they annihilate one another, disappearing in a puff of gamma rays. Futuristic scenarios for interstellar travel propose using antimatter as fuel, because its reaction with matter releases energy so efficiently.
One puzzle about antimatter remains. Theory suggests that the newborn universe had equal amounts of matter and antimatter, so why is matter so dominant today? Possibly, some slight symmetry allowed matter to win out. A more exotic possibility is that distant antimatter realms in the universe survive to this day, teeming with galaxies of antimatter stars.
Grand unified theories. Grand unified theories try to mathematically describe the forces of nature under one umbrella. Theory has unified the electromagnetic force and the weak force, showing that they acted like a single force in the hot, early universe, when particles were highly energetic. But to date, no satisfactory theory unites them with the strong force as well.
A satisfactory grand unified theory would explain various aspects of the standard model particles and forces that so far remain a mystery. For instance, why are there six quarks and six leptons? Why do they have their particular masses? The grand theories developed so far are unpleasantly complicated and invoke exotic, untested physics, some requiring space to have hidden extra dimensions.
The ultimate goal is to also unify gravity with the other forces in a “theory of everything”. One candidate is string theory, which assumes that particles are like tiny vibrating strings. But string theory has yet to make testable predictions to prove that it accurately describes nature’s design.
Atomic structure. Atoms consist of a tiny dense nucleus containing positively charged protons and uncharged neutrons, surrounded by clouds of electrons. Because protons and neutrons are much heavier than electrons, most of an atom’s mass resides in the central nucleus.
Each chemical element has a unique number of protons in its nucleus, its “atomic number”. For instance, the element carbon with six protons has the atomic number six. However, single elements can have different numbers of neutrons in the nucleus. For example, carbon has three naturally occurring “isotopes” with six, seven or eight neutrons. The sum of protons and neutrons in an atom’s nucleus is called the atomic mass number.
Normally, the net electric charge of an atom is zero because the number of electrons is the same as the number of protons, and their equal and opposite electric charges cancel out. However, it’s possible to knock electrons out of atoms or add extra ones to create positively or negatively charged “ions”.
Atomic nucleus. The atomic nucleus is the dense cluster of protons and neutrons that sits at the centre of an atom, surrounded by clouds of electrons. Nuclei are tiny compared to atoms themselves. If an atom was scaled up to the size of a football stadium, its nucleus would typically be about the size of a pea.
The atom’s structure was unclear until 1909, when a famous experiment by New Zealand physicist Ernest Rutherford showed that positive charge is densely concentrated in the middle. His team fired positively charged alpha particles at a thin sheet of gold foil and found that most flew through the foil in a straight line. However, a tiny number bounced off at larger angles. Rutherford realised these had happened to hit a tiny, positively charged nucleus at an atom’s centre.
Atomic nuclei are now known to be made up of protons and neutrons. The protons feel repulsion from each other due to their positive electric charge, but the attractive strong force between the protons and neutrons overcomes this repulsion to hold nuclei together.

Radioactivity. Radioactivity involves the spontaneous decomposition of an unstable atomic nucleus into a more stable type. There are three main types of decay, which were named simply “alpha”, “beta” and “gamma” in the days when they were poorly understood, and these names have stuck.
Alpha decay occurs when a heavy nucleus emits a particle containing two protons and two neutrons. Uranium-238 transforms into thorium-234, for instance, which has two fewer protons and two fewer neutrons. In beta decay, a neutron can convert into a proton with the emission of an electron, increasing the atomic number by one. Alternatively, an excited nucleus can emit a gamma ray.
Lead is the heaviest stable element, all heavier ones decaying over time. Radioactivity is a random, unpredictable process but the decay rates of many identical atoms can be reliably characterised by a “half-life”, the time it takes for half the nuclei to decay. Half-lives vary from a fraction of a second to many billions of years – longer even than the age of the universe.
Radioactivity techniques are commonly used during medical investigations and treatment, and in military operations were there has been nuclear fallout.

Nuclear fission and fusion. Nuclear fission occurs when a heavy atomic nucleus splits into two, with the release of (kinetic) energy. Nuclei are made up of protons and neutrons, but the mass of a nucleus is always less than the sum of the individual masses of the proton and neutrons inside. The difference is a measure of the “nuclear binding energy” that holds the nucleus together, and this energy is released when the nucleus splits. For example, Uranium-235 can split to form two lighter elements such as rubidium and caesium.
Nuclear fusion is the opposite process, in which two light nuclei merge to form a heavier one, yielding energy because the combination is lighter than the sum of its parts. Atoms heavier than iron can undergo fission, while lighter ones can fuse.
Nuclear power stations generate energy from fission reactions. About 2 billion years ago, natural fission took place at Oklo in Gabon, Africa, when groundwater concentrated uranium deposits. Fusion occurs in the Sun’s core, where hydrogen nuclei fuse into helium, generating the Sun’s energy.

This completes Science Book (Physics). Amendments to the above entries may be made in future.




